Learning intricate card tricks and complex hand movements can be overwhelming and hard to execute in front of an eager audience, so here is a how to perform a magic trick that does not require any skill but, instead, just a little bit of science. Here is the premise of the trick: You tell an unassuming bystander to pour a glass of water into your magician’s hat. You wave your hands over the hat and chant a seemingly realistic spell. When you flip the hat over, no water pours out… not even a drop. So how did this happen?
While the audience believes that the enchanting spell you cast over the hat made the water disappear, the real magic is rooted in the chemistry behind one particular item: the diaper. When we think of diapers, words like “ew” or “baby” or even “ew babies” come to mind. However, after reading this, you might have a new perspective on the amazing properties of diapers.
We’re now going to do something no ordinary person would do: dissect the diaper. The magical substance that you will line your magician’s hat with can be found underneath the lining of a diaper. This substance is a water-absorbing chemical called sodium polyacrylate. Polyacrylate can absorb more than 200 times its own mass (which is extremely fortunate for those potty training and those who have to change the diapers of potty trainers). Although this superabsorbent polymer is now found in a wide variety of products like medical bandages, female hygiene products and diapers, the substance was originally manufactured for the U.S. Department of Agriculture in the 1970s. The original use for polyacrylate gel was to improve the water retention in soils in an effort to improve germination and plant growth (Liu, 2001).
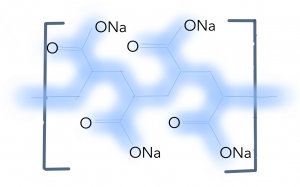
Sodium polyacrylate is a network polymer that contains sodium ions. The key to the absorption process and this magic trick is understanding basic rules of diffusion, osmosis and bonding. Osmosis is the when molecules in a solvent move from a less concentrated solution to a more concentrated solution. In this magic trick, when the water comes into contact with the sodium-containing polymer, the sodium diffuses into the water. This results in the replacement of sodium atoms with water molecules in the complex network (Carnegie Mellon University, n.d.). The polymer is essentially a long chain of negatively charged molecules and bonds to water through hydrogen bonds. The bonds allow the network to swell and hold the water in place, which is why the resulting mixture is crystal-like, fluffy substance that does not spill out of the hat. Now that you know the secret to this trick you will now be able to hone and perfect your water vanishing skills!
Hopefully now when you think of diapers you think “awesome, absorbent polymer.” That said, there are limitations to how amazing this polymer is. Although bunnies are about 70% water, please know a diaper will not make a bunny disappear.
Here’s what you need for the trick:
– Hat
– Source of sodium polyacrylate (>2g)
– Glass of water (8 floz.)
Start with some sodium polyacrylate at the base of the hat. You can tape a diaper to the bottom of the hat to conceal the water-absorbing polymer. Ask someone to pour a regular glass of water in the hat. Swirl the hat around and distract the audience while the water absorbs. The absorption time will depend on how much sodium polyacrylate you use and how much water is added, however, this process should occur within a matter of seconds to minutes. Once you are convinced everything has been absorbed, flip the hat over and watch the audience be amazed!
References:
Carnegie Mellon University (n.d.). Super absorbing polymer powder. Retrieved from https://www.cmu.edu/gelfand/education/k12-teachers/polymers/polymer-and-absorption/super-absorb-powder.html
Foy, J. M., & Schnieden, H. (1960). Estimation of total body water (virtual tritium space) in the rat, cat, rabbit, guinea‐pig and man, and of the biological half‐life of tritium in man. The Journal of physiology, 154(1), 169-176.
Liu, M., & Guo, T. (2001). Preparation and swelling properties of crosslinked sodium polyacrylate. Journal of Applied Polymer Science, 82(6), 1515-1520.
Parks, L. R. (1981). Cross-linked sodium polyacrylate absorbent. U.S. Patent No. 4,295,987. Washington, DC: U.S. Patent and Trademark Office.


