The Georgia Tech STEMcomm VIP team had the Taste of Science festival on March 12, 2018. During this event, my group (members from Georgia Tech SASE) and I decided to make dragon beard candy. Now let’s see the steps of making it and some science knowledge behind it.
corn starch, corn syrup, white sugar, vinegar, peanuts and sesame
Step 1
Pour 1kg white sugar, 100 g corn syrup, 2 cup of water, 2 tea spoon of vinegar into the pot and boil in medium fire for 30-40 min, do not stir!
Pour the caramelized syrup out and wait until it gets cool down. Put the candy into corn starch and start pulling. First of all, drill a holein the enter of candy to make it look like the shape of donut. Then make the hold bigger and start folding and double the strands.

Step 3
Tear down 5 cm of candy strands, put peanuts and sesame inside of the candy strands and wrap it up.
 During the process of making dragon beard candy, the most difficult step is the boiling part. Our first time, we didn’t understand the purpose of making caramelized syrup. Also, we didn’t know the function of the vinegar so we only used less than 1 teaspoon so we didn’t make our candy too sour. Then we boiled about 20 minutes and poured the sugar syrup out even though it didn’t change color. We even put it into refrigerator for 5 hours, but it was still too soft and sticky to make it into any shape.
During the process of making dragon beard candy, the most difficult step is the boiling part. Our first time, we didn’t understand the purpose of making caramelized syrup. Also, we didn’t know the function of the vinegar so we only used less than 1 teaspoon so we didn’t make our candy too sour. Then we boiled about 20 minutes and poured the sugar syrup out even though it didn’t change color. We even put it into refrigerator for 5 hours, but it was still too soft and sticky to make it into any shape.

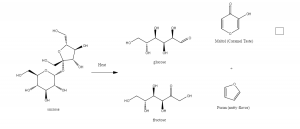
 After discussion, we decided to boil it for a longer time than the first time and surprisingly, we found the color of the syrup slowly changed to gold and yellow after 30 minutes, and then it turned into red and brown. Then we suddenly realized that the purpose of this step is to let the white sugar get caramelized.
After discussion, we decided to boil it for a longer time than the first time and surprisingly, we found the color of the syrup slowly changed to gold and yellow after 30 minutes, and then it turned into red and brown. Then we suddenly realized that the purpose of this step is to let the white sugar get caramelized.
White sugar is actually sucrose. When we heat sucrose solution, it first gets boiling. Keep heating, thermal decomposition reaction happens. Sucrose decomposes into glucose and fructose. At a high temperature, caramelization happens. According to the science of cooking, “ Caramelization is the oxidation of sugar and a type of non-enzymatic browning reaction. As the process occurs, volatile chemicals are released producing the characteristic caramel flavor. The reaction involves the removal of water (as steam) and the break down of the sugar. The caramelization reaction depends on the type of sugar. And the caramelization temperature about 160 C, 320 F. ” [1] Thus, the reason why we failed the first time is that we didn’t boil for long enough.
What’s more, based on the sugar property study, the inversion reaction is accelerated by the presence of acids (low pH) or by some enzymes. This reaction will accelerate with increasing acidity and increasing temperature. [2] This tells us why we should put 2 teaspoons of vinegar in order to accelerate the chemical reaction.
Citation
[1] http://www.scienceofcooking.com/caramelization.htm
[2] Knecht, R. L. “Properties of sugar.” Sugar (1990): 46-65.






