The Science of Smell
Of the five senses, scientists understand the least about smell. We recognize scents when small airborne molecules stimulate receptors in the back of our nose, which then send signals to our brain. Humans have about 500 different types of receptors, each one specific to a different scent molecule (“How the nose decodes complex odors”, 2020).
But what makes some molecules have scents? Well, the molecules must be able to travel through the air to the back of the nose, so they have to be volatile. Volatility is how easily the molecule evaporates at room temperature. The more a molecule evaporates into the air, the more likely it is that it reaches the receptors in the back of our nose. Even then, there must be a receptor for that specific molecule for it to be recognized (Gang, 2005).
The whole process seems simple enough, but most scents are a combination of tens to hundreds of different scent molecules. The smell of chocolate and cocoa products, for example, is made of over 600 scent molecules (Counet et al., 2002). Scientists still don’t fully understand how more complicated scents work, (Gottfried & Wilson, 2011) but most have been found to be made of just a few major compounds, such as the southern magnolia flower. Its scent comes from a mixture of 14 different molecules, but two, beta-caryophyllene and beta-cedrene, are the main compounds (Garg & Kumar, 1999).

Many common scents can be traced back to specific molecules. Even small changes in a molecule can cause large changes in its scent. Check out a few familiar scents below!
Common Scent Molecules
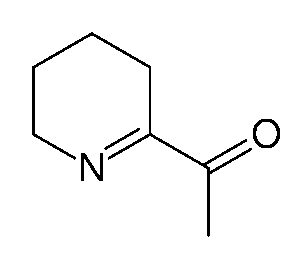
6-Acetyl-2,3,4,5-tetrahydropyridine gives popcorn and baked goods their typical smell. A slight change creates 2-Acetyl-1-pyrroline, which is the main component of rice’s smell (Dhrubo, 2015).

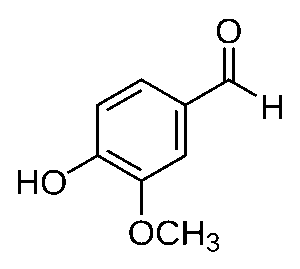
4-Anisaldehyde is used a lot in the fragrance industry. It has a very strong sweet floral scent and is very similar to vanillin. Vanillin, as you may have guessed, is the main component of vanilla’s scent. Natural vanilla extract is made of hundreds of different molecules, but synthetic vanilla scent is usually just pure vanillin (Dhrubo, 2015).

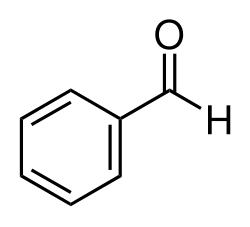
Benzaldehyde has an almond-like odor and is very useful in the fragrance industry because of its simple structure (Dhrubo, 2015).
Cinnamaldehyde makes up for around 90% of the scent of cinnamon (Dhrubo, 2015).
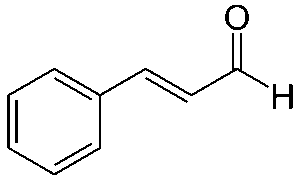
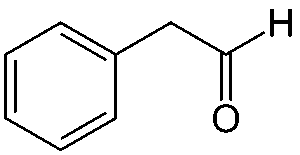
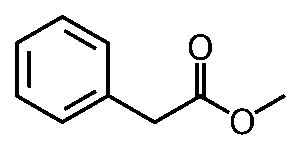
Natural honey is an extremely complicated scent, made up of 187 volatile compounds, and varies based on its nectar source (Mahmoud et al., 2024). Phenylacetaldehyde is the main component of honey’s scent, but methyl phenylacetate is used for a synthetic honey scent because of its strength. It only takes a little bit of methyl phenylacetate to make a perfume or candle honey-scented (Dhrubo, 2015).
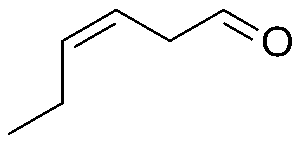
Even fresh cut grass has its own molecule. (Z)-3-hexenal is one of the molecules released when grass is damaged, and is what gives it that freshly mowed lawn smell (Dhrubo, 2015).
If you want to learn more about fun molecules, the American Chemical Society (ACS) has been publishing a “Molecule of the Week” since 2001. This past week’s molecule was myristicin, which is a primary component of many spices such as nutmeg, dill, parsley, and anise. Interestingly, its structure is very similar to that of amphetamines and can cause hallucinations if ingested in high doses (much higher than used for cooking). On their website, they have an archive of over one thousand molecules, complete with properties, history, and fun facts! Click here to check it out.
How the nose decodes complex odors. (May 12, 2020). National Institute of Health. https://www.nih.gov/news-events/nih-research-matters/how-nose-decodes-complex-odors
Gang, D. (June 2005). Evolution of Flavors and Scents. Annual Review of Plant Biology. 56, 301-325. https://www.annualreviews.org/content/journals/10.1146/annurev.arplant.56.032604.144128
Gottfried, J., Wilson, D. (2011). Neurobiology of Sensation and Reward. National Library of Medicine. https://www.ncbi.nlm.nih.gov/books/NBK92786/
Counet, C., Callemien, D., Ouwerx, C., Collin, S., (March 15, 2002). Use of Gas Chromatography−Olfactometry To Identify Key Odorant Compounds in Dark Chocolate. Comparison of Samples before and after Conching. Journal of Agricultural and Food Chemistry, 50(8), 2385–2391. https://pubs.acs.org/doi/10.1021/jf0114177
Garg, S., Kumar, S., (1999). Volatile constituents from the flowers of Magnolia grandiflora L. from Lucknow, India. Journal of Essential Oil Research 11(5),, 633-634. https://aromadb.cimapbioinfo.in/web_essentialoil_details.php?id=CRESOL159
Mahmoud, M., Kilic-Buyukkurt, O., Aboul Fotouh, M., Selli, S. (October 2024). Aroma active compounds of honey: Analysis with GC-MS, GC-O, and molecular sensory techniques. Journal of Food Composition and Analysis, 134(106545). https://www.sciencedirect.com/science/article/pii/S0889157524005799#:~:text=Compounds%20such%20as%20phenylacetic%20acid,the%20distinctive%20smell%20of%20honey.
Dhrubo, J. S. (2015) Esters, Terpenes And Flavours: Make The Mood Cheers by Three Musketeers!. World Journal of Pharmaceutical Medicine, 4(8), 1-40. https://wjpr.s3.ap-south-1.amazonaws.com/article_issue/1438667348.pdf


